New Molecular Profiling & Prior Auth for NSCLC
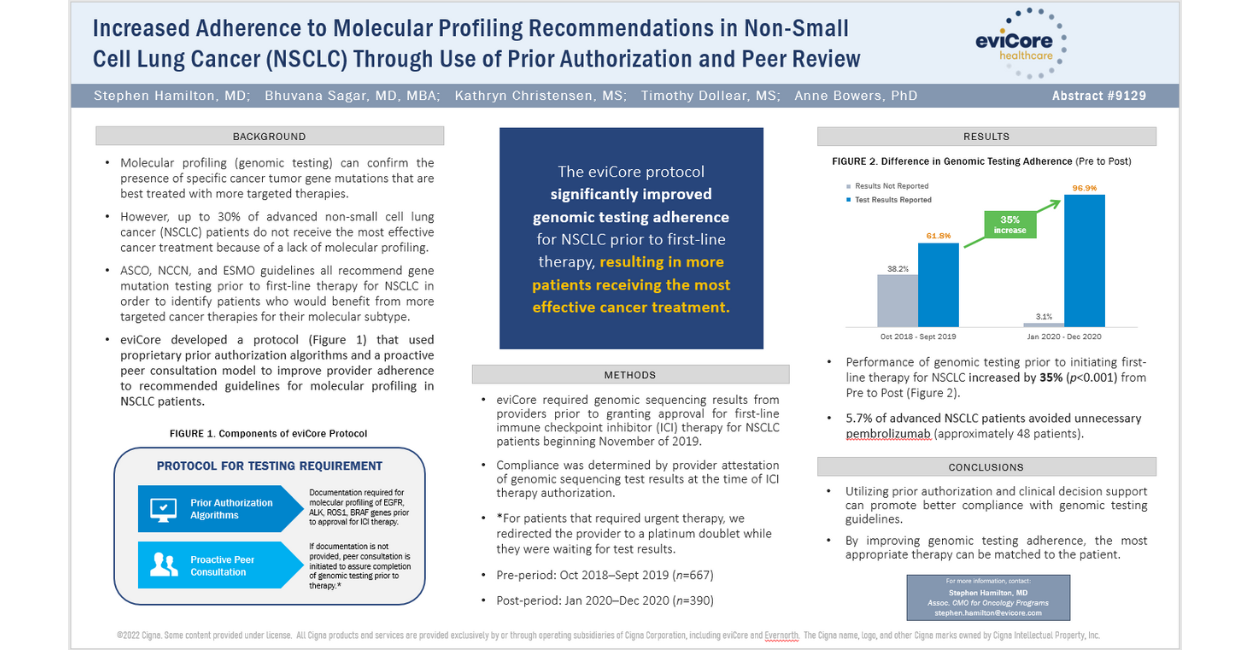
During the 2022 annual meeting of the American Society of Clinical Oncology (ASCO), EviCore’s Dr. Stephen Hamilton, Associate Chief Medical Officer for Oncology Programs, and colleagues Bhuvana Sagar, MD, MBA, Kathryn Christensen, MS, Timothy Dollear, MS, and Anne Bowers, PhD demonstrated our ability to improve genomic testing adherence for patients with advanced non-small cell lung cancer (NSCLC). Over 30,000 national and international oncology professionals attended this year’s conference, where Dr. Hamilton shared data showing how we effectively increased the appropriate use of genomic testing from 62% to nearly 100%.
ASCO is the world’s leading professional organization for doctors and oncology professionals who care for people with cancer. Research, plan of care, cancer care networking, and cancer education are just some of the resources that the ASCO provides to patients and professionals.
EviCore is applying the lessons of these findings to better serve health plans and patients and ensure our customers receive the best care possible. Our poster presentation showed that by utilizing our prior authorization platform and peer consultation with providers, we were able to improve guideline-recommended genomic testing in advanced NSCLC patients from approximately 62% to 97% after program implementation. This type of genomic testing is critical to selecting the optimal therapy for patients with advanced lung cancer. Hence, the improvement in genomic testing adherence we achieved helped many more patients receive the most effective therapy for their lung cancer.
To learn more about this type of testing and Dr. Hamilton’s presentation, please visit evernorth.com