
Sleep
Since 2008, EviCore's Sleep program has offered comprehensive management of sleep diagnostic procedures and positive airway pressure (PAP) therapy devices and supplies. Our approach prioritizes long-term adherence to PAP treatment, supporting patients in maintaining their therapy. Additionally, we manage other sleep apnea therapies, including oral appliances and hypoglossal nerve stimulators.
Sleep Diagnostics
EviCore currently manages medical necessity review through prior authorization (PA) for attended facility-based sleep studies. In the application of our Sleep solution, requests are evaluated against our evidence-based clinical guidelines and result in one of three outcomes:
- Approved if clinical guidelines are met.
- The requested sleep study is not authorized, but an alternative recommendation is provided, such as a different facility-based test, a home sleep study, or even immediate treatment when testing is not medically necessary.
- Denied if the information supplied does not meet criteria for any procedure requiring PA.
CPT codes for Sleep Studies
- Facility-based baseline polysomnography (PSG) for adults (95807, 95808, 95810) and children (95782)
- Split-night studies (95811)
- Facility-based PAP titration for adults (95811) and children (95783)
- Multiple sleep latency testing (95805)
- Facility-based Maintenance of Wakefulness Test (95805)
Sleep Apnea PAP Therapy
EviCore manages all durable medical equipment (DME) PAP therapy codes and supplies through medical necessity review and PA. Our PAP therapy program consists of four parts: initial PAP usage, confirmation of compliance with PAP therapy, PAP replacement machines, and ongoing resupply of PAP equipment. Our Sleep solution manages all of the following codes for PAP therapy:
- CPAP and Auto-CPAP Devices (E0601)
- Bi-level PAP Devices (E0470, E0471)
- PAP Therapy Masks and Supplies (A7027 – A7046)
- PAP Therapy Humidifiers (E0561, E0562)
TherapySupport PAP Compliance
EviCore’s system for monitoring participating members’ compliance with PAP therapy is called TherapySupportSM, which collects PAP usage and intervenes with DME and physician providers when patients are not meeting targeted usage levels. When a patient is not compliant with PAP therapy, a sleep educator contacts the DME provider and/or the treating physician to support effective treatment of obstructive sleep apnea. Our system enables health plans to efficiently obtain real-time, objective assessments of patient adherence to therapy.
Clinical Guidelines
EviCore’s evidence-based Sleep solution clinical guidelines are based upon major society guidelines and practice parameters, as well as national and international associations. In addition, peer-reviewed literature, major treatises, input from health plans, and practicing academic and community-based physicians are also utilized in the construction of our guidelines. EviCore’s Sleep clinical guidelines undergo an annual review process to ensure they reflect the latest evidence-based medicine.
American Academy of Sleep Medicine (AASM)
The American Academy of Sleep Medicine (AASM) recently awarded EviCore’s evidence-based clinical guidelines for diagnostic testing for obstructive sleep apnea with 5 stars. Across the nine criteria used, the AASM scored EviCore the highest, indicating that its guidelines are the most in line with the standard.
Sleep SmartChoice
EviCore’s innovative SmartChoice for Sleep program is a member outreach service focused on those members that have been diagnosed with sleep apnea but have not yet started PAP therapy. SmartChoice agents educate these health plan members on available sleep apnea treatment options and can get them scheduled with a DME provider. By working with these members, SmartChoice helps patients to overcome barriers and get critical therapy started.
Payment Integrity
Our integrated system Claims Studio delivers savings through an enhanced focus on accurate claims payment. The system can easily be made an extension of the client’s existing claims workflow.
Claims Studio
Our proprietary integrated system, Claims Studio, delivers savings through an enhanced focus on accurate claims payment. The system can easily be made an extension of the client’s existing claims workflow.
Frequently Asked Questions
Yes. EviCore operates prior authorization programs for current clients that cover all sleep diagnostic studies. Prior authorization can be required for all attended sleep studies, home sleep studies, and home APAP titration. Our experience shows that programs that include prior authorization for both attended and home studies improve the continuity of care a patient receives by linking each step of the process.
Communication with providers occurs before the Sleep solution is implemented and written notifications are delivered after every request for services or devices is completed. In addition, we monitor patients undergoing PAP therapy for sleep apnea treatment and our Sleep Educators communicate with the referring provider when the patient is not using therapy as prescribed.
Prior to the start of the Sleep solution, EviCore schedules webinars designed to communicate all elements of the Sleep solution to providers. Both the health plan and EviCore use mailed letters, fax, or email to notify providers to attend these presentations. After learning key facets of the solution, the providers are better equipped to secure authorizations for their patients.
In the medical necessity determination process, providers contact EviCore either by Web or phone to initiate requests for sleep diagnostic procedures, PAP therapy devices, and PAP therapy supplies. On the phone, providers or their staff speak with EviCore’s specially trained clinical decision support agents, who are specifically assigned to handle only Sleep solution cases. Whether the case is completed on the phone or on the Web, when it reaches a final determination (approved or denied) notification is immediately sent to the provider.
Philips Respironics is voluntarily recalling the below devices due to two (2) issues related to the polyester-based polyurethane (PE-PUR) sound abatement foam used in Philips Continuous and Non-Continuous Ventilators: 1) PE-PUR foam may degrade into particles which may enter the device’s air pathway and be ingested or inhaled by the user, and 2) the PE-PUR foam may off-gas certain chemicals. The foam degradation may be exacerbated by use of unapproved cleaning methods, such as ozone (see FDA safety communication on use of Ozone cleaners), and off-gassing may occur during initial operation and may possibly continue throughout the device’s useful life.
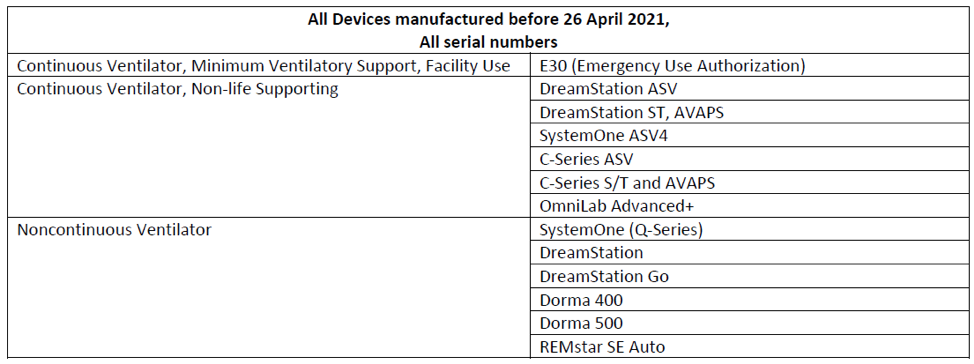
These issues can result in serious injury which can be life-threatening, cause permanent impairment, and/or require medical intervention to preclude permanent impairment. If you are a user of a product impacted by the recall, it is crucial you contact your doctor to discuss whether or not you should stop using your machine and you register your affected Philips Respironics devices at the following site: www.philips.com/src-update
- Sleep Diagnostics − EviCore currently manages medical necessity review and PA for attended sleep studies in a facility. In our programs, requests for attended sleep studies are evaluated against our nationally recognized evidence-based criteria.
- Sleep Apnea Therapy − EviCore manages all durable medical equipment PAP therapy codes and supplies through medical necessity review and PA. We also manage other treatments for sleep apnea when PAP therapy is not an option
- PAP Compliance − EviCore developed an innovative and effective system that monitors how well patients in sleep apnea treatment are complying with PAP therapy guidelines. Our system, called TherapySupport, objectively monitors PAP usage and intervenes with DME and providers when patients are not meeting targeted usage levels.
- Technology infrastructure − The Sleep solution resides on the same technology platform as all other EviCore solutions. Our technology infrastructure offers a wide range of flexibility in application and can accommodate a more streamlined request process for those providers who have demonstrated an inclination to order procedures in accordance with evidence-based medicine.
TherapySupport℠ was the first system to market an established direct connectivity to the PAP therapy manufacturers’ device reporting databases. TherapySupport℠ is a quality-of-care and patient-support activity aimed at improving adherence to prescribed treatments and decreasing the effects of sleep apnea-related comorbidities.
Currently, eviCore maintains clinical criteria for the following sleep procedures, PAP devices, and PAP supplies:
- Facility-based polysomnography for adults (95807, 95808, 95810) and children (95782)
- Split Night Studies (95811)
- Facility-based titration for adults (95811) and children (95783)
- Home Sleep Testing (95800, 95801, 95806, G0398, G0399, G0400)
- Multiple Sleep Latency Testing (95805)
- CPAP and APAP Devices (E0601)
- Bi-Level PAP Devices (E0470, E0471)
- PAP Therapy Masks and Supplies (A7027 thru A7046, A4604)
- PAP Therapy Humidifiers (E0561, E0562)
- PAP Therapy Compliance
- Oral Appliances (E0485, E0486, S8262)
During the last 40 years, medical professionals have come to recognize that getting a good night's sleep is critical to maintaining overall health and wellness. In fact, studies have found that sleep disorders contribute significantly to the incidence of high blood pressure, diabetes, obesity, heart failure, coronary artery disease, and stroke.
Through our prior authorization process, EviCore helps to direct at-risk, but medically uncomplicated patients to the more readily available and convenient option of home sleep testing, while directing the more complex patients to facility testing.
Welcome to part two of our Sleep Apnea series. This article covers treatment compliance, and treatment options.
Welcome to our sleep management team at EviCore. We know that quality sleep is fundamental to both physical and mental well-being. It plays a crucial role in maintaining overall health, supporting immune function, improving cognitive performance, and regulating mood. Inadequate sleep can exacerbate a range of physical and mental health conditions, such as cardiovascular disease, diabetes, depression, and anxiety.
Our dedicated team of physicians is passionate about helping providers effectively and efficiently diagnose and treat sleep disorders. Our guidelines are regularly reviewed in collaboration with leading academic experts in Sleep Medicine, thereby ensuring that they are based on the latest evidence. All of our physicians are board-certified in sleep medicine and bring expertise from diverse specialties, including anesthesia, pulmonary medicine, neurology, internal medicine, family medicine, and ENT. This multidisciplinary approach allows us to understand the unique needs of each individual.


